|
INTRODUCTION
The HMT (Heavy Metal Test) is easy to conduct and the results are not only quickly obtained but also provide a dramatic and convincing demonstration for the patient.
TEST DESCRIPTION
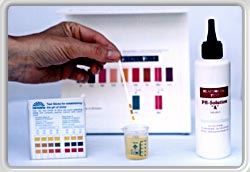 |
SETTING pH VALUE
For the test a morning urine specimen (about 30 ml) is required which, using the two pH Solutions A and B, is calibrated to the neutral value of 7.0.
|
 |
To begin with the test, place one of the dithizone treated indicator squares into a solution of Testsol, which is made from natural products.
The solution takes on a green coloration which signals phase 0 and constitutes the basis for all further tests. |
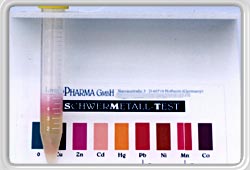 |
If now a liquid containing heavy metals is added (urine, saliva or other aqueous solution) these heavy metals react with the dithizone and the green coloration changes.
The new coloration is compared each time with those on the coloration chart on the test tube rack. Each coloration corresponds to a specific metal (zinc, copper, cadmium, lead, mercury etc.)
Depending the amount of tested liquid is added (2,4 or 6 ml) the concentration of the respective metal can be determined. (quantity testing)
|
The method permits the identification and relative concentration of several metals with only one reagent.
Detailed instructions on how to conduct the test and how to evaluate the results are contained in a booklet and in a pictogram.
|


